
NMN (nicotinamide mononucleotide) in patients with androgenetic alopecia (Hair loss)
Therapeutic effects of growth factor cocktail (CellcurinTM) containing FGF5s (fibroblast growth factor 5 short) and NMN (nicotinamide mononucleotide) in patients with androgenetic alopecia: A split study
Department of Dermatology, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
Department of Dermatology, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
Department of Dermatology, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
School of Systems Biomedical Science, Soongsil University, Seoul, Korea
DOI: 10.15761/GOD.1000229
Abstract
Background: Growth Factor Cocktail (GFC), which contains growth factors and cytokine, has been gradually gaining attention as a new treatment for hair loss. Several studies reported the effectiveness of GFC on hair regrowth via microneedles; however, studies regarding formulations with fibroblast growth factor 5 short (FGF5s) and nicotinamide mononucleotide (NMN) have not been conducted.
Objective: This study aimed to investigate the effect of treatment with microneedle technology by adding FGF5s and NMN to GFC in patients with androgenetic alopecia (AGA).
Methods: In this study, AGA patients were treated six times for a total of 3 months at 2-week intervals from the first visit. The scalp was divided into two sections at the time of treatment; the right side was treated with GFC with FGF5s and NMN, and the left were treated with normal saline, with microneedles to a depth of 0.8 mm. A total of 20 patients (11 males and 9 females) were included in the study. Clinical photographs and phototrichogram were performed before and 2 weeks after treatment to determine the final treatment effect.
Results: The phototrichogram images of the scalp treated with GFC for 3 months showed increased hair density and diameter. In contrast, there was no change in hair density and diameter in the scalp treated with normal saline.
Conclusion: GFC with FGF5s and NMN with microneedling is an effective and safe treatment of androgenetic alopecia. Future studies with more thorough investigations in controlled clinical settings are warranted.
Key words
androgenetic alopecia, CellcurinTM, fibroblast growth factor 9 short, growth factor cocktail, microneedle, nicotinamide mononucleotide
Introduction
Androgenetic alopecia (AGA) is a disease of the scalp that is common form of hair loss in both men and women. Recently, the oral administration of finasteride and dutasteride and topical application of minoxidil have been commonly used as treatment [1-3]. Furthermore, surgical methods such as scalp skin auto-implantation and follicle autograft have been implemented [1-3]. Recently, several studies on various cytokines contributing to the growth and differentiation of hair have been conducted, biochemical therapies different from conventional treatments for alopecia have been emerging. Accordingly, a growth factor cocktail (GFC) containing several growth factors and cytokines is currently being introduced. In addition to studies on GFC efficacy, we reported on the therapeutic effect of GFC formulations with FGF9 that promote the onset of growth within the hair cycle. The core aspect of this treatment is the transdermal absorption of GFC through a microneedle, which displays a therapeutic effect via absorption through the hair follicle. Fibroblast growth factor 5 short (FGF5s) prolongs the growth process by inhibiting the transition of the anagen to the catagen, while nicotinamide mononucleotide (NMN), a precursor of nicotinamide adenine dinucleotide+ (NAD+), is involved in cellular energy metabolism and helps promote hair growth. In this study, we investigated the effect of GFC with FGF5s and NMN into a depth of 0.8 mm using a microneedle.
Materials and methods
Patients
We selected 11 men and 9 women among the patients who visited the Alopecia Clinic, Department of Dermatology, Myongji Hospital. At the start, one man and three women dropped out due to protocol violation. The exclusion criteria for this study included receiving treatment for hair loss or taking medications that cause hair loss within a year, having severe seborrheic dermatitis, infectious or severe inflammatory skin lesions, keloid disease or collagen, elastic fiber disease, immunodeficiency disease, psychiatric problems, or undergoing treatments that can lead to an immune deficiency condition.
Materials and treatment regimens
All patients received treatment using a microneedle for intradermal absorption, and did not take any drugs or apply topical treatments for hair disease. The scalp was divided into two sections; the right side was treated with GFC with FGF5s and NMN and the left was treated with normal saline, using microneedles injected at a depth of 0.8 mm.
GFC: The GFC used in this study (CellcurinTM; PnP Biopharm, Seoul, Korea) consisted of insulin-like growth factor-1 (IGF-1, 2.5 µg/ml), stable acidic fibroblast growth factor (stable aFGF, 5 µg/ml), stem cell factor (SCF, 5 µg/ml), keratinocyte growth factor-2 (KGF-2, 5 µg/ml), fibroblast growth factor 9 (FGF9, 5 µg/ml), noggin peptide (10 µg/ml), protaetide (80 µg/ml), fibroblast growth factor 5-short (FGF5s, 10 µg/ml) and nicotinamide mononucleotide (NMN, 500 µg/ml).
Microneedle: The microneedle device (Raffine®; Woorhi Mechatronics, Anyang, Korea) consists of nine 33-gauge microneedles with automatic vertical movements (4200–6000rpm), and the needle depth is adjustable from 0.1 to 2.0 mm.
Treatment: GFC with FGF5s and NMN provided as a freeze-dried powder was dissolved in 5 ml normal saline before use. About 2.5 ml of the GFC solution was topically applied to the right side of the scalp, and 2.5 ml of normal saline on the left side of the scalp (Figure 1). Both sides were treated by a microneedle device at a depth of 0.8 mm. Each patient received six treatments at 2-week intervals for a period of 12 weeks.
Figure 1. Schematic diagram of the division of the scalp into right and left sides for the spilt test
Parameters measurement
A tattoo point was applied to the right and left side of the scalp of each patient before treatment to compare hair growth. A phototrichogram (Folliscope® 2.8; Lead M, Seoul, Korea) was taken from a fixed area marked with a tattoo on both sides to measure hair density and diameter at the baseline, and at the end of the treatment. An unbiased investigator, unaware of the study, counted the number of hairs and the hair diameter by using phototrichogram images.
Statistical analysis
All data are expressed as mean ± standard deviation. Wilcoxon matched-pairs signed-rank test was performed using STATA/SE ver.12 (Stata Corp LP, College Station, TX, USA) to test the effectiveness of the treatment on the same patients. The Student’s t-test was conducted to analyze the differences between the effect of the treatment on the right and left sides of the scalp. The statistical significance was calculated for p-values <0.05.
Results
Patient characteristics
Twenty out of 24 patients aged 22-47 years (mean, 35.9±6.5 years) completed a 12-week treatment course. The patients comprised: 11 male patients with a mean age of 37.5±6.4 years, and male pattern hair loss type Ⅱ (2 patients), type Ⅲ (6 patients), type Ⅳ (3 patients) according to the Hamilton-Norwood classification; 9 female patients with a mean age of 33.9±5.9 years, and female pattern hair loss type Ⅰ(6 patients), type Ⅱ (3 patients) according to the Ludwig scale (Table 1).
Table 1. Baseline characteristics of the study patients
|
|
|
Total |
Men |
Women |
|
Patients |
20 |
11 |
9 |
|
|
Mean age |
35.9±6.5 |
37.5±6.4 |
33.9±5.9 |
|
|
Pattern hair loss |
||||
|
MPHL II |
2 |
|||
|
MPHL III |
6 |
|||
|
MPHL IV |
3 |
|||
|
FPHL I |
6 |
|||
|
|
FPHL II |
|
|
3 |
|
MPHL: Male pattern hair loss; FPHL: Female pattern hair loss. |
||||
Treatment efficacy and adverse effects
The measurement was taken before and after treatment to compare the density and thickness of hair, respectively. In the phototrichogram images, the density and thickness of the hair increased on the right side of the scalp (Figure 2). The change of hair density and diameter between the right and left sides after treatment are shown in Table 2. On the right side of the scalp, hair density increased from 182.5±13.1/cm2 to 188.7±13.4/cm2(p-value<0.0001) and hair diameter increased from 62.1±9.3 µm to 64.05±9.2 µm (p-value<0.0112). On the left side, there were no significant differences; from 181.9±16.4/cm2 to 181.9±15.8/cm2(p-value=0.3650) in hair density, and from 62.1±8.3 µm to 62.4±8.7 µm (p-value=0.4076) in hair diameter (Figure 3). In addition, there were no adverse reactions related to the treatment except for minimal pain.
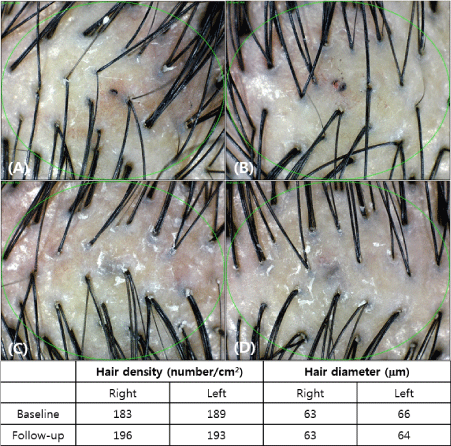
Figure 2. Evaluation of phototrichogram images after treatment for 12 weeks (A: Baseline, right side of the scalp; B: Baseline, left side of the scalp; C: After 12 weeks, right side of the scalp; D: After 12 weeks, left side of the scalp)
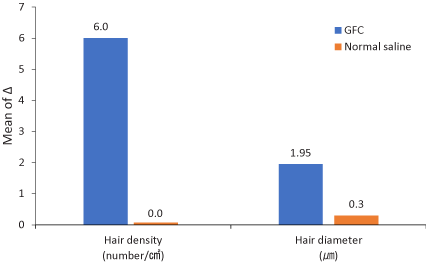
Figure 3. Change from baseline to 12 weeks after treatment. The graph shows that the increase of right-side parameters (GFC) is greater than that for the left-side parameters (normal saline). GFC: Growth factor cocktail
Table 2. Statistical analysis of the changes in hair density and diameter between baseline and 2 weeks after treatment
|
|
|
Baseline Mean±SD |
12 weeks Mean±SD |
Δ |
p-Value |
|
Hair density |
GFC |
182.5±13.1 |
188.7±13.4 |
6.2±0.43 |
<0.0001 |
|
(number/cm2) |
Normal saline |
181.9±16.4 |
181.9±15.8 |
0.0±0.3 |
0.365 |
|
Hair diameter |
GFC |
62.1±9.3 |
64.05±9.2 |
1.95±0.44 |
<0.0112 |
|
(µm) |
Normal saline |
62.1±8.3 |
62.4±8.7 |
0.3±0.2 |
0.4076 |
|
GFC: Growth factor cocktail; SD: Standard deviation; Δ: Change. |
|||||
Discussion
Androgenetic alopecia is the most common type of progressive loss of terminal hairs on the scalp in a characteristic distribution [4]. AGA is an androgen-dependent disease, and dihydrotestosterone (DHT) is thought to have the greatest effect on follicular changes [5]. DHT is a metabolite of testosterone and has a higher affinity for androgen receptors than testosterone. DHT acts on the dermal papilla of the hair where the androgen receptor and type 2 5-reductase are frequently expressed, which induces the miniaturization of hair follicles and converts terminal hair into vellus hair [6]. Although the cause of female pattern hair loss is still unclear, studies have shown that it is related to the male pattern hair loss, which is also partially contributed to by hormone imbalance [7]. In men, hair loss starts from the frontal line, followed by vertex thinning, and finally complete baldness, which falls under the Hamilton-Norwood classification. Female pattern hair loss appears as hair loss in the centro-parietal region while the frontal hairline is maintained and is classified as the Ludwig type. Currently, the most commonly used FDA-approved drugs for the treatment of hair loss are finasteride and topical minoxidil, while medical device based treatment includes low-level laser light therapy (LLLT), and meta-analysis for the effect of the treatment for each method showed a significantly higher therapeutic effect than the placebo group [1].
Hair follicles arise in developing skin due to a complex set of interactions that are likely to be mediated by diffusible, cell- and matrix-bound factors. Various hypotheses such as epithelial-mesenchymal interaction, extrafollicular growth-modulating signals such as hormones, growth factors, and other dietary changes, are introduced to the hair follicle cycle mechanism [8]. We have conducted several studies to date, focusing on the effect of various hair growth factors on hair follicles to treat hair loss. In this study, 7 growth factors (IGF-1, stable aFGF, SCF, KGF-2, FGF9, noggin peptide, and protaetide) that contribute to the growth of the previous hair were used in the form of basic solutions with the addition of FGF5s and NMN. IGF-1 has been shown to increase the number of hair follicles and prolong the growth phase [9]. Furthermore, it stimulates the proliferation of follicle cells in the anagen phase and downregulates the expression of transforming growth factor b1(TGF-b1) in hair follicles in a dose-dependent manner, thus preventing hair follicles from entering the catagen state [9]. SCF has been demonstrated to play a significant role in skin and hair pigmentation by activating melanocytes through the c-kit pathway [10]. Cultured dermal papilla cells obtained from patients with AGA secrete less SCF than normal cells, owing to the inhibition of SCF production by androgens in the dermal papilla [10]. KGF has been reported to protect the epidermis and hair follicles from cell death induced by ultraviolet irradiation, chemotherapeutic drugs, or cytotoxic agents [10]. FGF has been implicated in the control of epidermal and mesenchymal cell function, and it is likely that they also affect the proliferation and differentiation of cells in the cutaneous appendages during development [11]. Immunolocalization of the basic FGF (bFGF) adjacent to areas of proliferation in developing and in mature follicles suggests that this factor may regulate the mitotic activity of epithelial-derived cells; while acidic FGF (aFGF); appears in the differentiating cells of the follicle bulb and thereby might participate in the formation of structural components of the follicle or fiber [11]. FGF9 has been reported to be a triggering factor for Wnt expression and as a hair follicle regenerator [12]. Noggin is an antagonist of bone morphogenetic protein (BMP), which suppresses the transition of telogen to anagen [13]. Overexpression of noggin enlarges the size of anagen hair follicles [13]. FGF5s is a growth factor that promotes hair growth by blocking the activity of FGF5, which inhibits hair growth. It has been confirmed that FGF5s has an action that delays the anagen phase and upregulates factors important for hair growth such as IGF-1 and noggin [14-16]. NMN is a precursor of NAD+, and NAD+-dependent signaling plays an important role in the catabolic reactions and energy production in the cell [17]. NAD+ is an essential pyridine nucleotide that plays major roles in several critical biological processes, including oxidative phosphorylation and adenosine triphosphate (ATP) production, and the synthesis of cholesterol, fatty acids, and steroids [18].
Lee et al. [19] reported the effects of GFC with microneedling on short-term treatment in women with female pattern hair loss over a 5-weeks period. Our previous studies confirmed that GFC treatment effectively results in hair growth and thickening, and that the effect differed in accordance with the microneedle depth. Hair density increased by 17.5% in the scalp injected with GFC including bFGF, vascular endothelial growth factor (VEGF), KGF-2, SCF, IGF-1, superoxide dismutase-1 (SOD-1), and FGF9 at a depth of 0.8 mm and 4% in the scalp treated using normal saline [20]. Ro et al. [21] reported that the density and thickness of the hair significantly increased after applying a solution containing IGF-1, bFGF, VEGF, SCF, KGF-2, noggin peptide, and SOD-1 to a depth of 0.5 mm. Ro et al. [22] applied GFC including IGF-1, bFGF, VEGF, SCF, KGF-2, noggin peptide, SOD-1, FGF9, and FGF5s for 24 weeks using iontophoresis instead of microneedling and reported that the number of hairs significantly increased. Compared to the previous studies [20,21], FGF5s and NMN were added to the GFC, and the changes in the density of the hair were not significant in this study.
Recently, it has been suggested that microneedling facilitates the penetration of drugs and is one mechanism by which it promotes hair growth. The micro-injury caused by microneedling leads to minimal superficial bleeding and causes a wound healing cascade that releases of various growth factors, such as the platelet-derived growth factor (PGF), which transforms the growth factor-alpha and beta (TGF-α and TGF-β), connective tissue activating protein, connective tissue growth factor, and fibroblast growth factor (FGF) [23]. Repeated microneedle stimulation induces hair growth and increases Wnt3α, β-catenin, VEGF, Wnt10b mRNA and protein expression in a murine model [23]. The microneedle needs to be deep enough to penetrate the skin barrier for enhanced drug delivery, but also short enough to cause minimal skin injury and pain. Through previous studies, it was found that the appropriate depth is 0.5~0.8 mm; and therefore, the deepest depth in this range was selected in this study to obtain the maximum effect [20,23,24]. However, we observed no significant difference in hair density and thickness in the left scalp that was treated with normal saline.
The results of this study were less effective than the results from previous studies that used GFC with VEGF, which strongly induced perifollicular vascularization during the anagen phase. Since microneedling increases VEGF, the hair growth effect was greater when VEGF was used. In addition, the effect on the left scalp was minimal, which could be attributed to improper or weak transmission of power from the clinician to the scalp. Therefore, it can be concluded that the power of microneedling was also minor in the right scalp, and thus the hair density did not increase significantly in both the right and left sides. In addition, the hair density of participants in this study was higher than in previous studies, which suggests that there may have been a selection bias in our study. Therefore, further randomized controlled studies are needed. Other significant limitations of our study are the relatively small number of enrolled patients and the short study period. Moreover, the microneedling procedure may have errors depending on the skill of the clinician; the intensity level of the stimulations by the microneedle may have varied depending on the clinician's power. Eventually, studies with various injection depths and with controlled clinical settings are needed to determine the most effective microneedle depth.
Drugs used in AGA treatment have been reported to trigger various side effects. Finasteride deteriorates sexual function and contributes to erectile dysfunction, decreased libido, and ejaculatory dysfunction [25]. In addition, topical minoxidil has minor side effects, including contact dermatitis, irritant dermatitis and hypertrichosis of the face [26].
Several studies have demonstrated the usefulness of LLLT. Proposed mechanisms include the acceleration of cellular mitosis, the stimulation of hair follicle stem cells or follicular keratinocytes, effects to increased adenosine triphosphate production and cellular activity, and anti-inflammatory effects [27]. In our opinion, this therapy accompanied by GFC treatment seems to be an interesting option with few side effects for the treatment of AGA.
Conclusion
In this study, GFC with FGF5s and NMN is an effective treatment for hair regrowth and thickness in patients with AGA. Since this treatment has few adverse effects, it can be used for patients who are difficult to treat due to their sensitivity to side effects or corresponding contraindication to the drugs. Besides, the treatment has the advantage of being in combination with finasteride, minoxidil, or LLLT. This study confirmed the effectiveness of the treatment with GFC, which could be used in addition to AGA. However, additional studies involving many patients will be needed to confirm the effects of the treatment more accurately, and further evaluation will help determine the exact mechanism of the treatment.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
Cellcurin™ was provided by PNP Biopharm (Seoul, Korea). This research (2019-07-013) was performed as part of the Research Program for Clinical Professor of Myongji Hospital, Goyang 10475, Korea.
References
- Adil A, Godwin M (2017) The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol 77: 136‐141. [Crossref]
- Avram M, Rogers N (2009) Contemporary hair transplantation. Dermatol Surg 35: 1705‐1719. [Crossref]
- Leavitt M, Perez-Meza D, Rao NA, Barusco M, Kaufman KD, et al. (2005) Effects of finasteride (1 mg) on hair transplant. Dermatol Surg 31: 1268‐1276. [Crossref]
- Blume-Peytavi U, Blumeyer A, Tosti A, Finner A, Marmol V, et al. (2011) S1 guideline for diagnostic evaluation in androgenetic alopecia in men, women and adolescents. Br J Dermatol 164: 5-15. [Crossref]
- Inui S, Itami S (2013) Androgen actions on the human hair follicle: perspectives. Exp Dermatol 22: 168-171. [Crossref]
- Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE (1974) Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science186: 1213-1215. [Crossref]
- Smith MA, Wells RS (1964) Male-type alopecia, alopecia areata, and normal hair in women. Arch Dermatol 89: 155-158.
- Stenn KS, Paus R (1999) What controls hair follicle cycling? Exp Dermatol 8: 229‐236. [Crossref]
- Li J, Yang Z, Li Z, Gu L, Wang Y, et al. (2014) Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-ß1 in C57BL/6 mice in vivo. Growth Horm IGF Res 24: 89-94. [Crossref]
- Grichnik JM, Burch JA, Burchette J, Shea CR (1998) The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. J Invest Dermatol 111: 233-238. [Crossref]
- du Cros DL (1993) Fibroblast growth factor and epidermal growth factor in hair development. J Invest Dermatol 101: 106S-113S. [Crossref]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, et al. (2013) Fgf9 from dermal γδT cells induces hair follicle neogenesis after wounding. Nat Med 19: 916-923. [Crossref]
- Botchkarev VA, Botchkareva NV, Sharov AA, Funa K, Huber O, et al. (2002) Modulation of BMP signaling by noggin is required for induction of the secondary (Nontylotrich) hair follicle. J Invest Dermatol 118: 3-10. [Crossref]
- Li WR, He SG, Liu CX, Zhang XM, Wang LQ, et al. (2017) Ectopic expression of FGF5s induces wool growth in Chinese merino sheep. Gene 627: 477‐483. [Crossref]
- He X, Chao Y, Zhou G, Chen Y (2016) Fibroblast growth factor 5-short (FGF5s) inhibits the activity of FGF5 in primary and secondary hairfollicle dermal papilla cells of cashmere goats. Gene 575: 393‐398. [Crossref]
- Ito C, Saitoh Y, Fujita Y, Yamazaki Y, Imamura T, et al. (2003) Decapeptide with fibroblast growth factor (FGF)-5 partial sequence inhibits hair growth suppressing activity of FGF-5. J Cell Physiol 197: 272‐283. [Crossref]
- Pehar M, Harlan BA, Killoy KM, Vargas MR (2018) Nicotinamide adenine dinucleotide metabolism and neurodegeneration. Antioxid Redox Signal 28: 1652‐1668. [Crossref]
- Braidy N, Berg J, Clement J, Khorshidi F, Poljak A, et al. (2019) Role of nicotinamide adenine dinucleotide and related precursors as therapeutic targets for age-related degenerative diseases: rationale, biochemistry, pharmacokinetics, and outcomes. Antioxid Redox Signal 30: 251-294. [Crossref]
- Lee YB, Eun YS, Lee JH, Cheon MS, Park YG, et al. (2013) Effects of topical application of growth factors followed by microneedle therapy in women with female pattern hair loss: a pilot study. J Dermatol 40: 81-83. [Crossref]
- Ro BI, Lee SY, Kim JB, Shin HC (2017) Therapeutic effects of growth factor cocktail including fibroblast growth factor 9 in patients with pattern hair loss. Korean J Dermatol 55: 504-511.
- Ro BI, Son HO, Chun SW, Shin HC (2016) Therapeutic effects of growth factor cocktail treatment in patients with androgenetic alopecia according to the depth of microneedle. Korean J Dermatol 54: 184-190.
- Ro BI, Kim SM, Kang JW, Shin HC (2020) Effect of iontophoresis with growth factor cocktail containing fibroblast growth factor 5-short applied to the scalp in patients with androgenetic alopecia - A split study. Glob Dermatol 7: 2-4.
- Kim YS, Jeong KH, Kim JE, Woo YJ, Kim BJ, et al. (2016) Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol 28: 586-592. [Crossref]
- Ro BI, Chun SW, Lee SY, Shin HC (2016) Therapeutic effects of growth factor cocktail including fibroblast growth factor 9 in patients with androgenetic alopecia – a split study. Glob Dermatol 3: 404-408.
- Mella JM, Perret MC, Manzotti M, Catalano HN, Guyatt G (2010) Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol146: 1141-1150. [Crossref]
- Ebner H, Müller E (1995) Allergic contact dermatitis from minoxidil. Contact Dermatitis 32: 316-317.
- Jimenez JJ, Wikramanayake TC, Bergfeld W, Hordinsky M, Hickman JG, et al. (2014) Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: a multicenter, randomized, sham device-controlled, double-blind study. Am J Clin Dermatol 15: 115-127. [Crossref]




